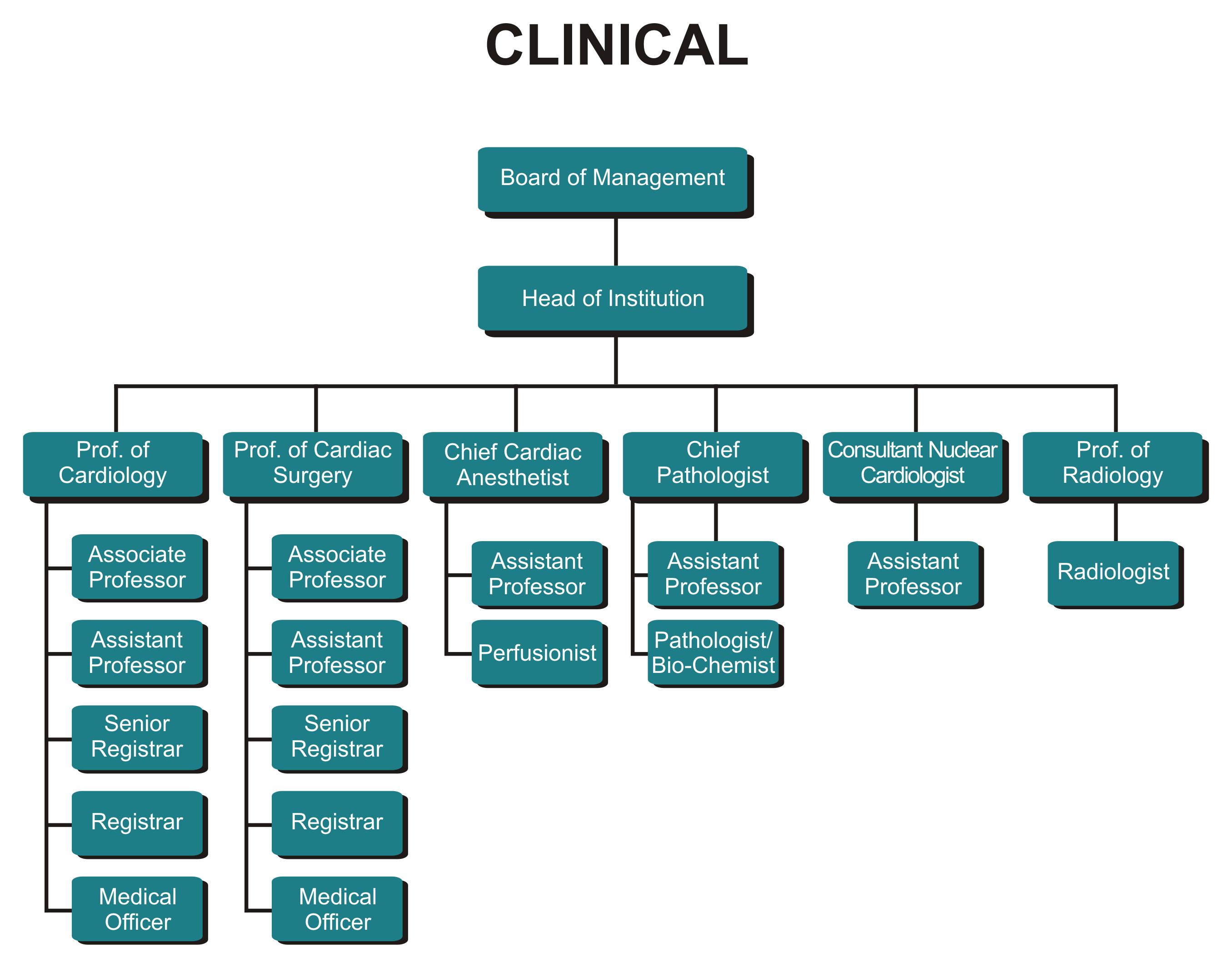
Organogram In Medical Laboratory
Abstract Preview
ISO 15189:2012 specifies requirements for quality and competence in medical laboratories.
National Strategic. Adolescent and youth ART National HIV Clinical Support Centre Adult ART Laboratory Monitoring & Coordination TB/HIV and Integration Units Voluntary Male Medical Circumcision Supply Chain & Pharmacovigilance Nutrition Department Division Sections Global Fund MOH-CDC COAG Procurement Finance & Account Human.
ISO 15189:2012 can be used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies.
General information
- Publication date : 2012-11
Corrected version (fr) : 2014-08 - Number of pages : 53
- :Clinical laboratory testing and in vitro diagnostic test systems
- ICS :
- Quality management and quality assurance
- Laboratory medicine in general
Buy this standard
en| Format | Language |
|---|---|
| std1pdf_colorpdf_epub178 | PDF + Color PDF + ePub |
| std2pdf178 | |
| std3pdf_epub_redline214 | PDF + ePub + Redline |
| std4paper178 | Paper |
Life cycle
A standard is reviewed every 5 years
- 10Proposal
- 30
- 40Enquiry
- 50
- 60Publication
- 90.92

Revisions / Corrigenda
- Previously
ISO 15189:2007 - Now under review
ISO 15189:2012 - Will be replaced by
ISO/CD 15189
Opening hours:
Monday to Friday - 09:00-12:00, 14:00-17:00 (UTC+1)
Keep up to date with ISO
Sign up to our newsletter for the latest news, views and product information
Geartrax 2014 full crack. Create superior designs and artwork. Intuitively create movies and video content.